We recently hosted a Twitter chat with Damon Runyon Clinical Investigator, Joshua Brody, MD of the Icahn School of Medicine at Mount Sinai to discuss his latest breakthrough - a promising new cancer vaccine that activates the immune system to fight tumors throughout the body. In a small clinical trial, lymphoma patients went into full remission for months or even years with this treatment. While Dr. Brody didn't have the time to answer all the questions about his exciting research during the hour-long Twitter chat, the full interview is presented here. To stay up-to-date on breaking cancer research news, sign up for our newsletter to the right of this post.
1. What treatments are currently available for lymphoma?
There are different types of lymphomas. Low-grade lymphomas are mostly treated with chemotherapy and anti-CD20 therapy. These are good, but patient's cancer generally recur, & disease becomes resistant. These lymphomas are usually incurable with standard treatments.
2. Why is this called a cancer vaccine—is it preventative?
Great question! MOST vaccines we think about are preventative (polio, measles, etc). It's important to clarify that this is a THERAPEUTIC vaccine, it teaches your immune system to recognize the problem AFTER you already have it.
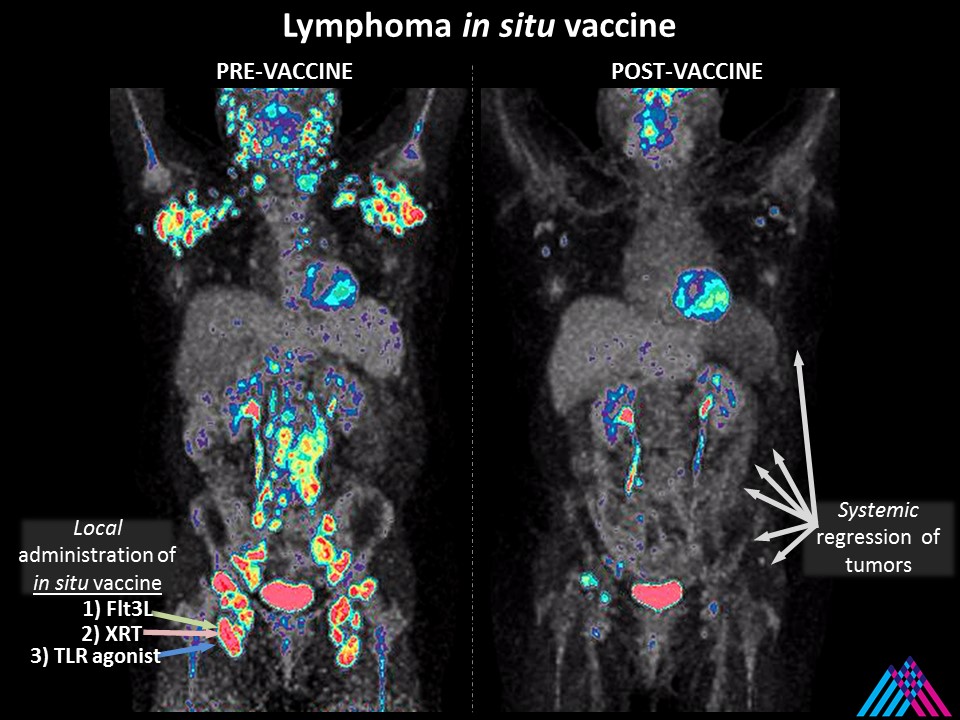
3. How is this treatment able to target tumors throughout the body?
Like other vaccines that protect the WHOLE body from infections, the in situ vaccine induces dendritic cells (generals of the immune army) to teach T cells (immune soldiers) to recognize tumor features (antigens) and eliminate them throughout the body.
4. How does this treatment target cancer cells while leaving healthy cells unharmed?

5. What advantages does this approach have over other immunotherapies such as CAR T and checkpoint inhibitors?
CAR T and checkpoint inhibitors are both miraculous therapies for certain groups of patients. So far, in our patients, the cancer vaccine has the advantage of being safer than those therapies.
6. What are you most excited about with the results of this clinical trial?
We saw patients with really advanced stage, extensive burden cancer who had profound regressions of nearly all their tumors. View the before and after video.
7. There is a lot of excitement around the cancer vaccine and the Nature Medicine paper. Are there opportunities available for other scientists and postdocs to get involved in this research?
Absolutely. We are currently hiring post-docs and research associates to work on these projects. Anyone interested can get in touch with me here on Twitter or on LinkedIn.
8. Could this approach work for other solid tumors, which ones?
Yes. The concept already works well in melanoma in lab studies. Now, we are making the vaccine available to patients with breast cancer or head/neck cancer. Learn more about our clinical trial.
9. Why did you decide to do a follow-up trial testing a combination of this treatment with a checkpoint inhibitor?
Some vaccinated patients’ T cells expressed the checkpoint –PD1 (a sign of T cell ‘exhaustion’), adding a PD1-blocking antibody may re-invigorate those T cells. In lab studies, although PD1 blockade cured 0% of large tumors => adding the vaccine increased the cure rate to 75%.
10. Are there any concerns about side effects?
There is always a concern with new therapies. In theory, whenever we push the immune system to kill cancer, we MIGHT push too hard and the immune system could attack healthy cells. We have not seen that with the in situ vaccine, but we will watch closely.
11. Were you surprised by the positive outcome of this research direction?
I would say delighted, but not surprised. We’ve known for years that dendritic cells control much of the immune system, the discovery won the 2011 Nobel Prize. So, loading DC with tumor antigens SHOULD teach the immune system to reject cancer.
12. What has been the most common reaction to the results of the clinical trial?
The most common reaction is patients reaching out from around the world asking if they are eligible for the cancer vaccine. So far, we can only treat patients with lymphoma, breast, and head/neck cancers. Hopefully we can help more people in the future.







