Chimeric antigen receptor (CAR) T cell therapy, in which a patient’s own immune T cells are genetically engineered to target their cancer cells, is one of the most promising advances in cancer therapy of the past decade. Having demonstrated the effectiveness of CAR T cells against a range of blood cancers, researchers now seek to design CAR T cells that can remain active in the body for longer and more efficiently eliminate tumors, with the goal of reducing costs and bringing CAR T therapy to more patients.
At the University of California, San Francisco, former Damon Runyon Fellow Kyle G. Daniels, PhD, and his colleagues are using machine learning to guide the design of next-generation CAR T cells. Their latest findings, published in Science, may hold the key to engineering longer-lasting, more powerful anti-tumor immunity.
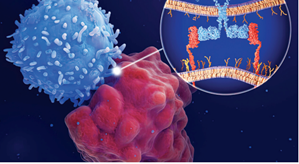
As the word “chimeric” suggests, chimeric antigen receptors are made from multiple parts: one domain that binds to specific proteins (known as antigens) on the surface of the patient’s cancer cells, and another that binds to signaling molecules that trigger an immune attack against the cancer. This second domain is itself composed of different building blocks, or “signaling motifs,” that determine what kind of immune response will occur. Some signaling motifs, for example, trigger a signaling cascade that leads to increased T cell memory and persistence, while others induce more effective T cell killing but reduced long-term T cell persistence. As Dr. Daniels writes, “Signaling motifs can be thought of as the ‘words’ that compose the ‘sentences’ communicated through signaling domains.” The goal, then, is to find out what sentence the immune system most needs to hear.
To this end, Dr. Daniels and colleagues created a library of CARs with over 2,300 different combinations of 13 signaling motifs, including hundreds of novel “sentences.” They observed how each type of CAR T cell responded to leukemia cells in the lab—specifically, how effectively the T cells killed the cancer cells and how long they remained active before becoming exhausted. By feeding this data into machine learning software, the team was able to elucidate the “grammar” of signaling motifs and identify some key design rules to induce a stronger immune response (the molecular equivalent of adding “please”!)
With the set of rules provided by Dr. Daniels and his team, labs around the country are better equipped to design the CAR T cells of the future, cells that can penetrate solid tumors, induce cancer remission, and produce long-lasting anti-tumor immunity. We have evidence that the immune system is capable of meeting such demands—it’s just a matter of finding the magic words.







