The outsider advantage: how extrachromosomal DNA fuels cancer
A basic tenet of biology is that our DNA is stored in chromosomes, that familiar grid of Xs (and sometimes Ys) containing all the instructions for the creation and maintenance of a human body. Another tenet of biology is that all rules have exceptions. As early as the 1960s, a group of scientists began noticing extra little rings of DNA in cancer cells. They called these “double minutes”—“double” because the rings were found in pairs, and “minute” because they were minuscule—and deemed them a rare and likely insignificant exception to the rule.
Sixty years later, a series of groundbreaking discoveries has quashed this premature assumption. Extrachromosomal DNA (ecDNA), we now know, is neither rare nor insignificant in cancer, and it plays by an entirely different set of rules.

At the forefront of these discoveries has been former Damon Runyon Scholar Howard Y. Chang, MD, PhD, and his lab at Stanford (from which he is currently on leave, having accepted the position of Chief Scientific Officer at Amgen last year). In 2018, Dr. Chang’s lab was studying chromatin, the packaging material that allows two meters of DNA to fit into a cell’s nucleus. Because our DNA is packed so tightly, much of our genome is not accessible to the cell’s transcription machinery, which allows genes to be “expressed.” Consider a manual that is stapled closed—the instructions are all there, but no one can read them, much less execute them. To identify which parts of our genome are expressed, Dr. Chang’s lab developed a sequencing tool that signals where the chromatin packaging is “open.” To their surprise, the most readily accessible DNA was not located on our chromosomes at all. The loudest “transcribe me!” signal, they found, was coming from ecDNA.
Around the time of this discovery, a scientist from the University of California, San Diego, came to Stanford to present his work. In his lab at UCSD, Paul S. Mischel, MD, had been tracing cancer-causing genes to their location within the genome, and he, too, had been surprised by his findings. Contrary to the popular conception that one or two percent of tumors contained ecDNA, Dr. Mischel was tracing oncogenes back to these little rings in about a third of all tumors—and some of the most well-known oncogenes, including EGFR, KRAS, and MYC, were found on ecDNA half the time.
Sitting in the audience, Dr. Chang’s hunch grew more certain: ecDNA played a far bigger role in cancer than anyone realized. He invited Dr. Mischel to his office that very afternoon.
Their first joint paper, in 2019, showed that ecDNA gives cancer cells more than just a place to store copies of their tumor-fueling genes. In addition to causing a “copy number problem,” as this hypothesis suggests, these rings bestow a gene expression advantage: every copy of an extrachromosomal DNA sequence generates four times the messenger RNA as the exact same sequence on a chromosome.
“Imagine that we’re in an argument,” Dr. Chang explains. “And for every sentence you say, I get to say four sentences. I would probably end up winning that argument, right? That’s what the cancer cell is doing by invoking this extrachromosomal DNA mechanism.”
By the time Damon Runyon Fellow Xiaowei Yan, PhD, joined the Chang lab in 2021, the phenomenon of ecDNA had caught the attention of cancer researchers around the world. Dr. Yan was eager to join the rapidly developing investigation of “double minutes,” as she first heard them termed.
“They were mentioned on one page of my genetics textbook in school,” she recalls. “I remember thinking, what a concept, but there was no more information about it.”
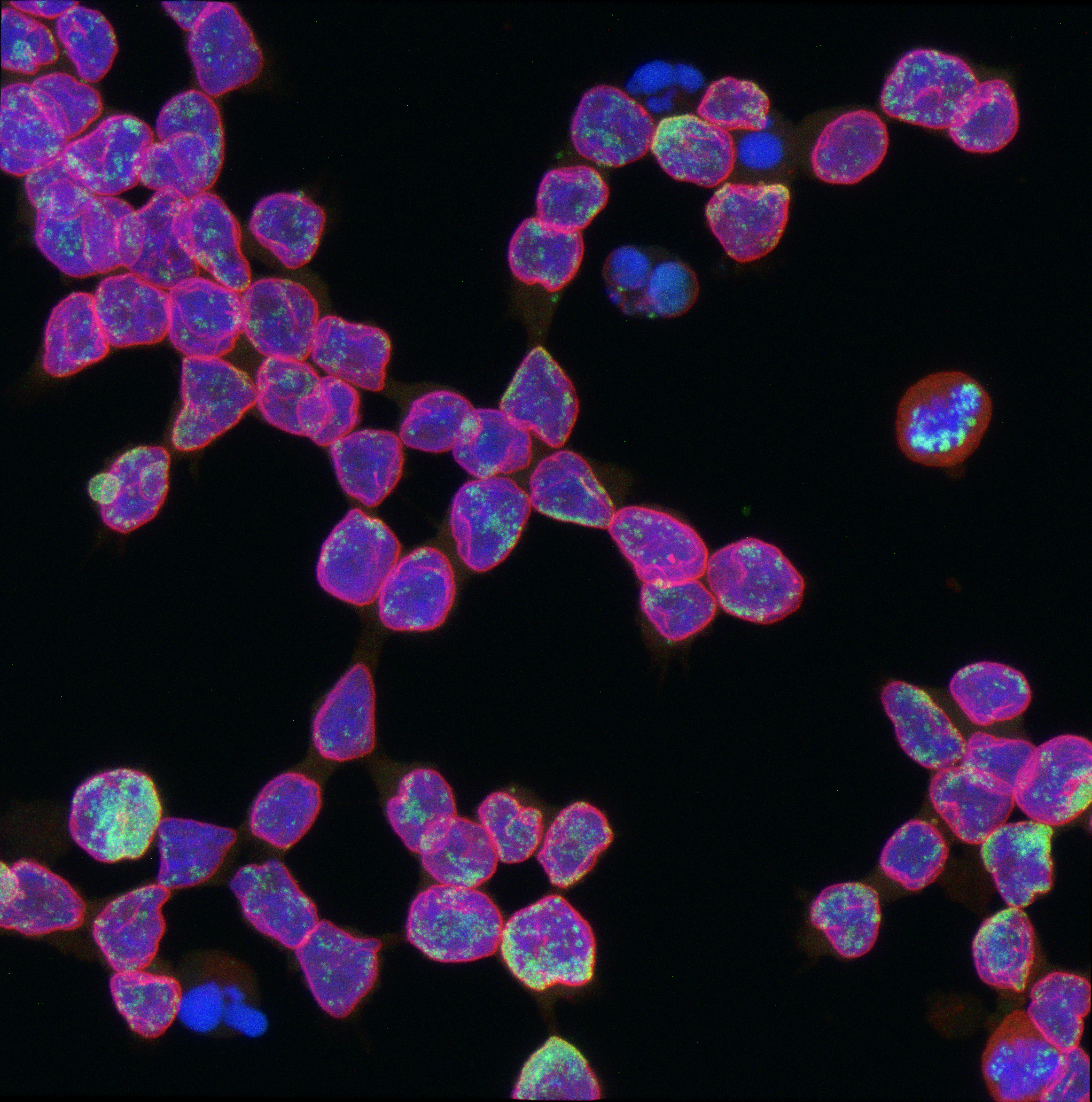
Dr. Yan arrived with expertise in microscopy technology, with which she studies the spatial organization of molecules inside cells. Using these skills, she helped the Chang lab observe and track ecDNA dynamics in live cells. This led to the discovery of yet another advantage ecDNA grants cancer cells—namely, exemption from the laws of genetics.
According to Mendel’s law of independent assortment, genes on different chromosomes are distributed between daughter cells independently of one another; receiving gene A from chromosome C makes the cell no more likely to receive gene B on chromosome D. But ecDNAs, the team observed, tend to be inherited together. If a daughter cell has ecDNA A, it likely also has ecDNA B. Sometimes it has even more copies of the oncogene than the parent cell.
Again, Dr. Chang is ready with an analogy. “If you get some nice cards in poker, imagine how awesome it would be if the next deal you get those great cards again, over and over,” he says. “You're going to keep winning.”
Clearly, these anomalous features of ecDNA give cancer cells a competitive edge over healthy cells. But Dr. Chang and his colleagues are hopeful that they also present a therapeutic vulnerability. For example, ecDNA’s increased rate of DNA transcription also confers higher risk of DNA damage in these cells. Inhibiting an enzyme called CHK1, which cells need to fix DNA damage, has shown promise as a means of targeting ecDNA-containing cells.
As a Damon Runyon alumnus and former member of the Foundation’s Innovation Award Committee, Dr. Chang is attuned to the potential of basic science inquiries, when properly supported, to translate into clinical impact for cancer patients.
“Damon Runyon is successful because it invests in all stages of the discovery process, including very early ideas about fundamental biology,” he says. “This business about whether DNA chromatin is open or closed—it was a basic gene regulation question, but the curiosity and the technology developed to address that question led to this insight about eCDNA in cancer. When we evaluate a project proposal, we have to think about the broadness of impact in the fullness of time.”
A basic biologist herself, Dr. Yan is grateful that Damon Runyon espouses this view.
“I need to understand something well before I have faith that I can change it,” she says. “That's how I feel about cancer. We need to understand the mechanism before we can interfere with it.”
This article was first published in the Fall 2025 issue of Damon Runyon's donor newsletter, Momentum.
