Yuxuan Chen, PhD

Dr. Chen is genetically re-engineering cancer-infecting viruses so that, once inside a tumor cell, they flip on a built-in “self-destruct” circuit called pyroptosis. This explosive form of cell death not only wipes out the infected cell but also broadcasts an alarm that rallies the immune system against the whole tumor. By pairing this viral upgrade with an ultrasound trigger, Dr. Chen aims to turn treatment-resistant pancreatic cancer—and, ultimately, other solid tumors—into diseases the immune system can eradicate. Dr. Chen received his PhD and MS from Zhejiang University, Hangzhou, and his BS from Southwest Jiaotong University, Chengdu.
Srivatsan Raghavan, MD, PhD

Pancreatic cancer is a highly lethal disease with relatively few treatment options. A new class of inhibitors that target the KRAS gene, which is altered in approximately 90% of pancreatic cancer patients, are showing great promise in the clinical setting as a new therapeutic option for these patients. However, nearly all patients develop resistance and experience tumor regrowth after a relatively short period of treatment with these drugs. Dr. Raghavan aims to investigate how cancer cells adapt and become resistant to these KRAS inhibitors and develop combination therapies to overcome this resistance. He anticipates that these studies will uncover fundamental mechanistic insights into cancer drug resistance and identify novel therapeutic strategies that will improve outcomes for patients with pancreatic cancer.
Fangyu Liu, PhD

Dr. Liu’s research focuses on discovering new drug candidates to treat pancreatic, colorectal, breast, and prostate cancers. Using advanced computational techniques to screen billions of chemical compounds, she aims to identify and develop highly specific molecules that target critical pathways in cancer cells while sparing healthy tissues. For example, she has uncovered compounds that modulate calcium-sensing receptors, which play a role in certain cancers, with reduced side effects compared to the current standard-of-care. She is now applying these insights to improve treatments that boost immune responses against tumors. Dr. Liu’s work not only strives to create new cancer therapies but also deepen our understanding of the complex interactions within tumors, paving the way for precision medicine tailored to individual patients.
Kashish Jain, PhD

A majority of pancreatic cancer cases harbor a mutation in the KRAS gene, which is involved in cancer initiation, progression, and chemotherapy resistance. Drugs targeting KRAS mutations are often met with resistance due to limited drug penetration into the tumor. Since pancreatic cancer progression involves increased tissue stiffening, KRAS signaling might be controlled by tissue stiffness. Dr. Jain [Damon Runyon-Lois A. Cinelli Awardee supported by the Cinelli Family Foundation] is studying the mechanisms that underlie tissue stiffness-dependent KRAS signaling at the molecular level. Understanding these mechanisms will uncover new ways to block aberrant KRAS signaling or reduce the effects of tissue stiffness on cancer progression, ultimately informing new combination therapies with KRAS-targeting drugs. Dr. Jain received his PhD from the National University of Singapore, Singapore and his BTech and MTech from Indian Institute of Technology, Kanpur.
Isabella Evavold, PhD

Pancreatic cancer remains unresponsive to current chemotherapy and immunotherapy treatments. However, with the recent development of mRNA vaccines and drugs that target cancer cell mutations, there is hope for a new generation of immune-based therapies. The ability of adaptive immune cells, called cytotoxic T cells, to kill cancer cells is central to anti-tumor immunity. Using mouse models of human pancreatic cancer, Dr. Evavold [Merck Fellow] plans to identify the flags presented by cancer cells that enable T cells to recognize them as foreign and kill them. One category of flags that label cancer cells as foreign may be proteins from bacteria that prefer to replicate within the tumor environment. This investigation of cancer cell targets will inform the development of future vaccines to treat cancer and prevent tumor regrowth or metastases. Dr. Evavold received her PhD from Harvard University, Cambridge and her BS from Emory University, Atlanta.
Nalin Ratnayeke, PhD

Pancreatic cancer is a leading cause of cancer-related deaths. The development of drugs targeting mutant KRAS, the oncogenic driver of most pancreatic cancers, has led to much optimism for improved treatments. However, tumor recurrence driven by heterogeneous cancer cell responses to these drugs remains a major challenge. Some cancer cells die, while surviving cells can halt their proliferation or continue to proliferate in the presence of drug, all of which can occur within the same tumor and dictate the overall response to treatment. Dr. Ratnayeke [HHMI Fellow] is studying the mechanisms that underlie these heterogeneous responses using mouse models of pancreatic cancer and single-cell genomics to map cellular states to their drug responses. Understanding these mechanisms will inform combination and precision therapies with mutant KRAS-targeting drugs to tune tumor responses in beneficial directions. Dr. Ratnayeke received his PhD from Stanford University, Stanford and his BS from the University of Texas at Austin, Austin.
Steven M. Corsello, MD

Pancreatic cancer is a devastating disease with limited treatment options. New strategies are urgently needed, but few actionable therapeutic targets are known. By systematically testing diverse molecules against pancreatic cancer cells combined with gene knockout studies, Dr. Corsello [Leslie Cohen Seidman Clinical Investigator] has identified a starting point to simultaneously activate inflammatory signaling and cell death pathways. He will determine the efficacy and underlying molecular mechanism of this approach, and potential immunotherapy combinations, using patient-derived tumor models. His goal is to accelerate the development of more effective and less toxic therapies for pancreatic cancer.
Ahmed Roman, PhD
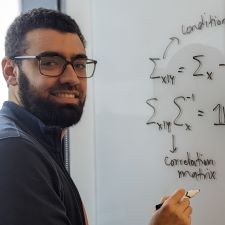
Dr. Roman [Leslie Cohen Seidman Quantitative Biology Fellow] aims to develop mathematical tools to determine which genes are associated with resistance to chemotherapy. Given genomic information from pancreatic cancer patients whose tumors are resistant or sensitive to chemotherapy, this tool will identify genes that distinguish the two populations. These genes can then be explored as potential drug targets that can sensitize chemotherapy-resistant tumors to treatment.
Dr. Roman’s research relies on the use of information theory to improve the ability of neural networks to find genes whose RNA expression distinguishes chemotherapy-sensitive from resistant patients. Another research direction is to leverage prior knowledge, accumulated over decades about gene-gene interactions in the laboratory, to inform the architecture of the neural networks or use large foundation models training on millions of cells to study cancer.
Isabella Fraschilla, PhD

Pancreatic cancer remains unresponsive to current chemotherapy and immunotherapy treatments. However, with the recent development of mRNA vaccines and drugs that target cancer cell mutations, there is hope for a new generation of immune-based therapies. The ability of adaptive immune cells, called cytotoxic T cells, to kill cancer cells is central to anti-tumor immunity. Using mouse models of human pancreatic cancer, Dr. Fraschilla [Merck Fellow] plans to identify the flags presented by cancer cells that enable T cells to recognize them as foreign and kill them. One category of flags that label cancer cells as foreign may be proteins from bacteria that prefer to replicate within the tumor environment. This investigation of cancer cell targets will inform the development of future vaccines to treat cancer and prevent tumor regrowth or metastases. Dr. Fraschilla received her PhD from Harvard University, Cambridge and her BS from Emory University, Atlanta.
Erik Van Dis, PhD

The innate immune system is the body's first line of defense against pathogens. The innate immune sensor MDA5 detects nucleic acids derived from pathogenic genomes or damaged cells and drives the production of cytokines, an important signaling molecule in the immune inflammatory response. MDA5 can be aberrantly activated by host nucleic acids, however, leading to autoimmune activation. Hyperactive MDA5 alleles are associated with the development of autoimmune diabetes. Dr. Van Dis [Robert Black Fellow] aims to define the innate immune signaling pathways that initiate autoimmune diabetes to better understand immune activation pathways in the pancreas and guide the development of novel immunotherapies for pancreatic cancer. Dr. Van Dis received his PhD from the University of California, Berkeley and his BA from Carleton College, Northfield.
