Translocation renal cell carcinoma (tRCC) is a rare but aggressive type of kidney cancer that disproportionately affects women and children. These cancers arise when part of a chromosome breaks off and fuses to a different chromosome, an event known as translocation. In tRCC, the fusion occurs between genes in the MiT/TFE family, which code for proteins called transcription factors that turn other genes on or off. Beyond this, however, the molecular basis of the disease is poorly understood. Due to this cancer’s rarity, doctors have an incomplete picture of its clinical features and no established standard of care. As a result, patients with tRCC are treated with therapies developed for other kidney cancers, with uneven success.
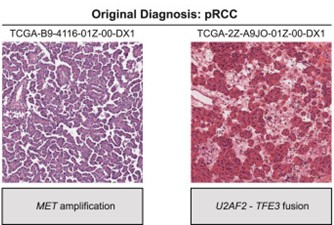
One barrier to studying tRCC is that, under a microscope, the tissue resembles other kidney cancers and is often misclassified. But at Dana-Farber Cancer Institute, former Damon Runyon Clinical Investigator Eliezer M. Van Allen, MD, and current Damon Runyon-Rachleff Innovator Srinivas R. Viswanathan, MD, PhD, have turned this obstacle into an advantage. By reviewing existing genomic and clinical trial datasets, they were able to retroactively identify enough tRCC cases to describe the molecular and clinical hallmarks of this enigmatic cancer.
Analyzing their found samples alongside current patient samples, the researchers observed that tRCC tumors are “genomically quiet,” with few genetic mutations apart from the MiT/TFE fusions. This finding clarifies the genetic underpinnings of other molecular features of tRCC. For example, the team noticed heightened activity in the NRF2 signaling pathway, which helps cells survive under chemical stress such as that caused by chemotherapy. The absence of other discernible mutations suggests a direct link between MiT/TFE fusions and the NRF2 pathway.
In turn, heightened NRF2 signaling may explain a third important finding: tRCC patients were more likely to respond well to immunotherapy than to chemotherapy. This was surprising, given how “quiet” tRCC tumors are—the immune system is usually better at fighting cancers that draw attention to themselves with highly mutated cells. Again, the answer seems to lie with the MiT/TFE fusion; the gene abnormality produces mutant proteins that immune cells recognize as threatening, while the increased NRF2 activity helps the tumors defend themselves against the chemical stress of chemotherapeutic drugs.
With this detailed picture of its molecular and clinical landscape, Dr. Van Allen and Dr. Viswanathan have brought tRCC out from the shadow of other kidney cancers, generating multiple hypotheses for further investigation. They have also identified exciting opportunities for possible therapeutic intervention, including NRF2 inhibitors, drugs that target the few other recurring genetic mutations found in the tumor samples, and personalized immunotherapies.
This research was published in Cell.







