Ras proteins, present in all mammalian cells, are molecular switches that control the processes of cell survival and proliferation. Unsurprisingly, mutations in any of the three RAS genes (KRAS, NRAS, or HRAS) can lead to uncontrolled cell growth, or cancer. Since these cancer drivers were first identified in the 1980s, it has been clear that different types of cancer are coupled with specific RAS mutants. For example, nearly 90% of pancreatic tumors display KRAS mutations, while NRAS mutations are more likely to appear in blood cancers. Why these associations exist, however, is not well understood.
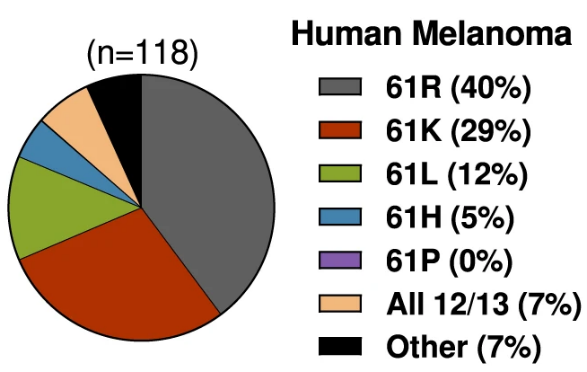
of NRAS-driven melanomas
An early hypothesis was that cause of the cancer determined which RAS mutant was linked to it. In some cases, this is true—carcinogens from cigarettes cause KRAS mutations in lung cells, so lung cancers are associated with KRAS mutants. But in other cases, that logic falls apart. The most common NRAS mutations in melanoma, for instance, are not directly caused by exposure to UV light. Given these inconsistencies, a new hypothesis has emerged: that each RAS mutant fulfills different requirements for tumor development, depending on the tissue.
So, is it the mutation-causing event or some property of the mutant proteins that explains the RAS-cancer associations? This is the question former Damon Runyon Innovator Christin E. Burd, PhD, set out to answer with her colleagues at The Ohio State University. Examining eight different NRAS mutants found in melanoma, the team found that the three most common mutant proteins have certain unique structural features that facilitate tumor development. These findings support the latter hypothesis: that differences between the mutant proteins, rather than UV-driven mutation, determines which NRAS mutants appear in melanoma.
Crucially, the team’s findings also elucidate the mechanism by which these three NRAS mutants drive melanoma tumor development. The structure of these proteins allows them to bind and activate another molecular switch, known as BRAF, better than other mutants. Once bound, BRAF sends the signaling pathway responsible for cell growth and proliferation into overdrive.
With a better understanding of why certain NRAS mutants are linked to melanoma, researchers can now target functions exclusive to these proteins, opening the door for better therapeutic and preventative strategies. In the meantime, keep slathering on sunscreen; UV light is not off the hook.
This research was published in Nature Communications.







