Twenty-two brilliant early career investigators will receive funding to pursue cutting-edge cancer research.
The Damon Runyon Cancer Research Foundation, a non-profit organization focused on supporting brilliant, early career researchers, named 16 new Damon Runyon Fellows. The recipients of this prestigious, four-year award are outstanding postdoctoral scientists conducting basic and translational cancer research in the laboratories of leading senior investigators across the country. The Fellowship encourages the nation's most promising young scientists to pursue careers in cancer research by providing them with independent funding ($231,000 total) to work on innovative projects.
The Committee also named six new recipients of the Damon Runyon-Dale F. Frey Award for Breakthrough Scientists who will each receive $100,000 toward their research. This award provides additional funding to scientists completing a Damon Runyon Fellowship Award who have greatly exceeded Damon Runyon’s highest expectations and are most likely to make paradigm-shifting breakthroughs that transform the way we prevent, diagnose and treat cancer.
2020 Recipients of the Damon Runyon-Dale F. Frey Award for Breakthrough Scientists:
 Lindsay B. Case, PhD (Damon Runyon Robert Black Fellow ‘16-’19) University of Texas Southwestern Medical Center
Lindsay B. Case, PhD (Damon Runyon Robert Black Fellow ‘16-’19) University of Texas Southwestern Medical Center
Dr. Case is investigating the mechanisms that regulate focal adhesion formation, growth, physical properties and subsequent downstream signaling. Focal adhesions are large protein complexes that connect the cell cytoskeleton to the extracellular membrane, which is the connective material holding cells in place. She is using a unique in vitro system in parallel with live cell imaging and cellular perturbations to dissect the specific molecular interactions that contribute to integrin signaling and focal adhesion function. Dr. Case’s research has the potential to identify new strategies for disrupting integrin signaling in cancer, which may provide a deeper understanding of how multivalent interactions and protein phase separation regulate cellular communication with the external environment.
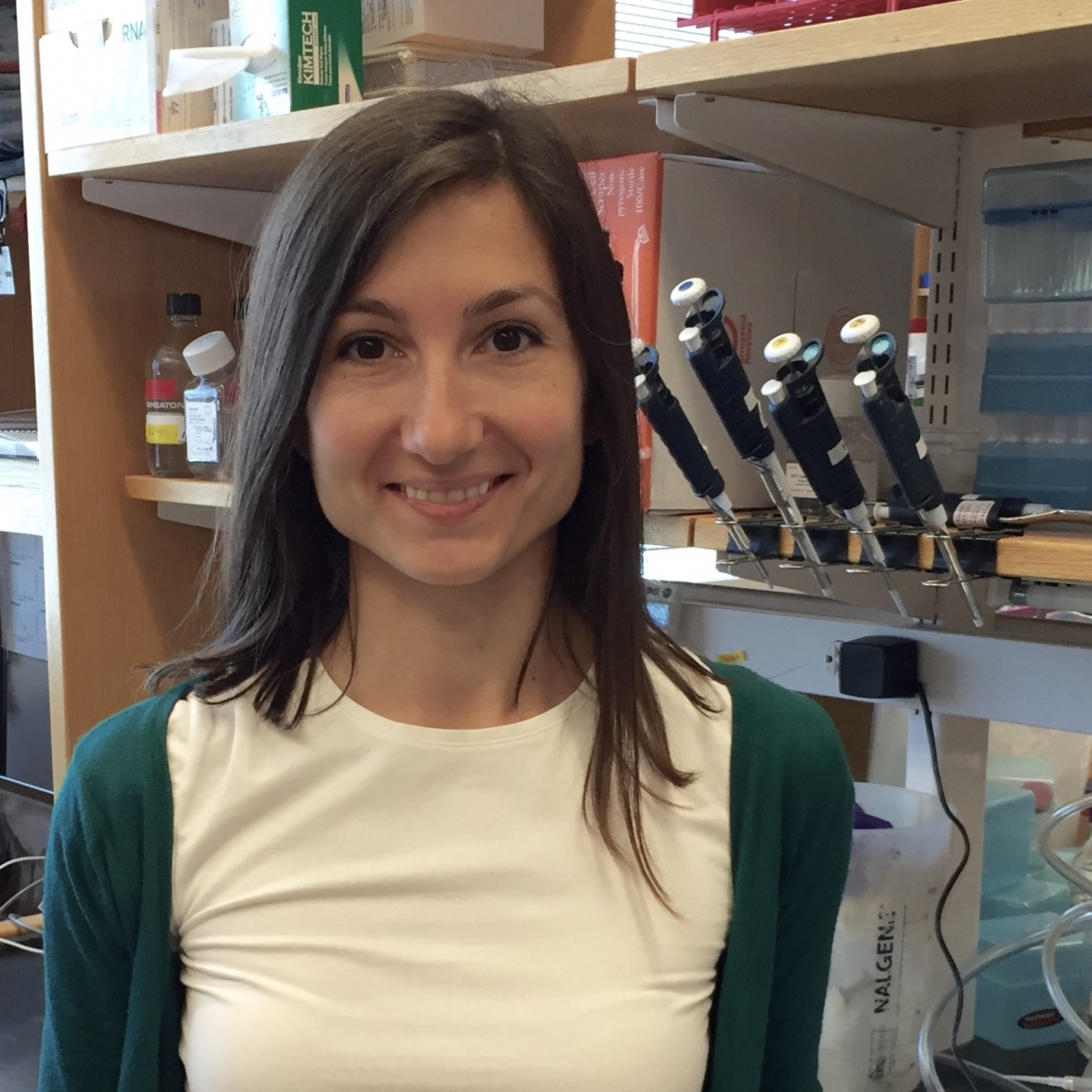 Ivana Gasic, PhD (Damon Runyon Merck Fellow ’16-’20) Harvard Medical School
Ivana Gasic, PhD (Damon Runyon Merck Fellow ’16-’20) Harvard Medical School
Dr. Gasic is investigating the fundamental principles of tubulin autoregulation and the Microtubule Integrity Response, a mechanism that detects and responds to changes in the microtubule cytoskeleton in interphase cells. Microtubules are frequent chemotherapy targets when treating various cancers, such as leukemia, lymphomas, melanoma, lung, ovarian, and breast cancer. Microtubule-targeting chemotherapeutics kill cancer cells by blocking cell division and inhibiting cell growth. Dr. Gasic’s work reveals how the microtubule-targeting drugs may work, independently of the cell cycle, and suggests potential new pathways to specifically target cancer cells.
 Natasha M. O’Brown, PhD (Damon Runyon Fellow ’16-’20) Harvard Medical School
Natasha M. O’Brown, PhD (Damon Runyon Fellow ’16-’20) Harvard Medical School
Dr. O’Brown is investigating the molecular mechanisms that govern the blood-brain barrier (BBB) which acts as the gatekeeper for the brain. While the BBB protects the brain from pathogens and maintains the environment for normal brain function, the BBB also acts as an obstacle to drug delivery for neurological diseases, including brain tumors. Dr. O’Brown has established the timeline of zebrafish BBB functional development using reporter lines and tracer assays. She is using CRISPR technology to create mutants that will shed light on details of BBB biology. A better understanding of the molecular determinants underlying BBB properties may provide new therapeutic avenues for drug delivery.
 Benjamin M. Stinson, PhD (Damon Runyon Fellow ’16-’20) Harvard Medical School
Benjamin M. Stinson, PhD (Damon Runyon Fellow ’16-’20) Harvard Medical School
Dr. Stinson focuses on a cellular DNA repair process called non-homologous end joining (NHEJ), which repairs most DNA double-strand breaks (DSBs) in vertebrates. Failure to repair DSBs properly can lead to new mutations, which are a central feature of cancer initiation and progression. Dr. Stinson has discovered one way the NHEJ machinery modifies DNA at DSBs and minimizes errors when re-joining broken ends of the DNA molecule. He will further investigate the basic mechanisms of the two main DSB repair pathways, which are critical to understanding the causes of many cancers and guiding therapeutic approaches.
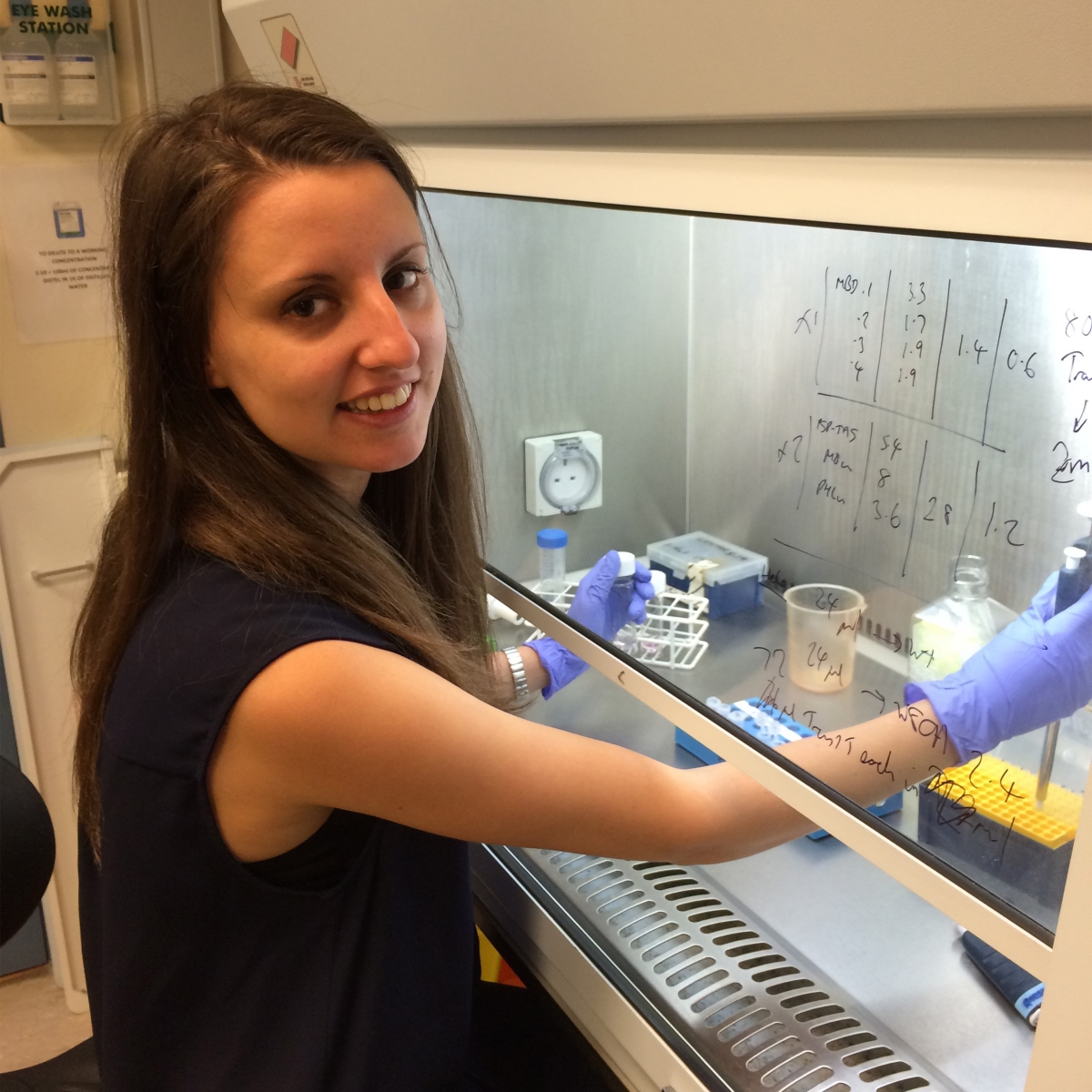 Iva A. Tchasovnikarova, PhD (Damon Runyon Fellow ’16-’20) Massachusetts General Hospital
Iva A. Tchasovnikarova, PhD (Damon Runyon Fellow ’16-’20) Massachusetts General Hospital
Dr. Tchasovnikarova has developed an unbiased, broadly applicable method to identify cell-based reporters of any epigenetic process inside the nucleus. Epigenetic modifications do not change the DNA sequence, but affect which genes are turned on or off, ensuring that only necessary proteins are produced. Disruption of epigenetic regulation, a hallmark of cancer, can result in malignant cellular transformation. Dr. Tchasovnikarova will utilize her novel technology to better understand the fundamental biology underlying these epigenetic mechanisms and identify optimal targets for novel therapies.
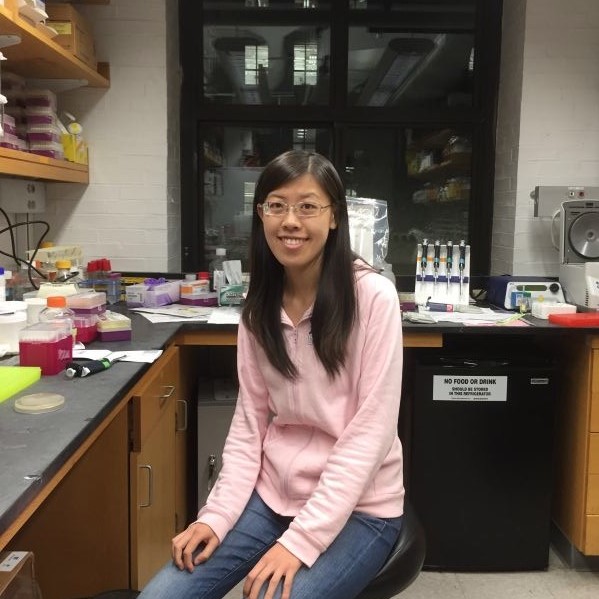 Yi Yin, PhD (Damon Runyon Fellow ’16-’19) University of California, Los Angeles
Yi Yin, PhD (Damon Runyon Fellow ’16-’19) University of California, Los Angeles
Dr. Yin has developed single-cell assays that will be combined with statistical modeling to understand homologous recombination (HR). Cells use the process of HR to accurately repair harmful breaks that occur on both strands of DNA. Failure to correct such DNA damage can play a role in cancer initiation and progression. Dr. Yin aims to understand this critical mechanism to help guide treatment approaches for many cancer types.
November 2019 Damon Runyon Fellows:
 Tess C. Branon, PhD [Robert Black Fellow], with her sponsor Gregory M. Barton, PhD, at the University of California, Berkeley, is exploring the relationship between the human body and the microbes that inhabit the gut, which affects physiology, development and disease. Recently, scientists discovered that cancer patients with a greater abundance of the bacteria Akkermansia muciniphila in their guts respond better to checkpoint inhibitor immunotherapies. Dr. Branon is using transcriptomic and metabolic profiling, as well as genetic manipulation of both the host and microbe, to elucidate the molecular interactions that underlie this protective effect.
Tess C. Branon, PhD [Robert Black Fellow], with her sponsor Gregory M. Barton, PhD, at the University of California, Berkeley, is exploring the relationship between the human body and the microbes that inhabit the gut, which affects physiology, development and disease. Recently, scientists discovered that cancer patients with a greater abundance of the bacteria Akkermansia muciniphila in their guts respond better to checkpoint inhibitor immunotherapies. Dr. Branon is using transcriptomic and metabolic profiling, as well as genetic manipulation of both the host and microbe, to elucidate the molecular interactions that underlie this protective effect.
 Zibo Chen, PhD, with his sponsor Michael Elowitz, PhD, at the California Institute of Technology, Pasadena, is creating the molecular language of cell signaling from the bottom up. Intra- or extra-cellular signals vary continuously and are often interpreted combinatorially in cells. Neural networks in biology and computer science offer a powerful way to interpret signal combinations. Dr. Chen will combine protein design and synthetic biology approaches to build a protein-based cellular circuit that can sense multiple inputs and carry out diverse functions based on pre-programmed instructions. This work will create a powerful “molecular computing” model that may provide insight into biological systems and open new capabilities for personalized cell-based therapies.
Zibo Chen, PhD, with his sponsor Michael Elowitz, PhD, at the California Institute of Technology, Pasadena, is creating the molecular language of cell signaling from the bottom up. Intra- or extra-cellular signals vary continuously and are often interpreted combinatorially in cells. Neural networks in biology and computer science offer a powerful way to interpret signal combinations. Dr. Chen will combine protein design and synthetic biology approaches to build a protein-based cellular circuit that can sense multiple inputs and carry out diverse functions based on pre-programmed instructions. This work will create a powerful “molecular computing” model that may provide insight into biological systems and open new capabilities for personalized cell-based therapies.
 Steven W.M. Crossley, PhD [AGBT-Elaine R. Mardis Fellowship in Cancer Genomics], with his sponsors Christopher J. Chang, PhD, and Daniel K. Nomura, PhD, at the University of California, Berkeley, is developing reaction-based chemistry to address the problem of “undruggable” targets in cancer therapy. These proteins lack grooves or “pockets” on the surface that can potentially bind small molecule drugs. A small molecule that can react with an amino acid in the target protein, forming a covalent bond, may circumvent the problem of poor binding pockets. Dr. Crossley aims to find drugs that will target the most problematic cancer-causing proteins, expanding the potential to treat cancers that currently have few therapeutic options.
Steven W.M. Crossley, PhD [AGBT-Elaine R. Mardis Fellowship in Cancer Genomics], with his sponsors Christopher J. Chang, PhD, and Daniel K. Nomura, PhD, at the University of California, Berkeley, is developing reaction-based chemistry to address the problem of “undruggable” targets in cancer therapy. These proteins lack grooves or “pockets” on the surface that can potentially bind small molecule drugs. A small molecule that can react with an amino acid in the target protein, forming a covalent bond, may circumvent the problem of poor binding pockets. Dr. Crossley aims to find drugs that will target the most problematic cancer-causing proteins, expanding the potential to treat cancers that currently have few therapeutic options.
 Erin E. Duffy, PhD, with her sponsor Michael E. Greenberg, PhD, at Harvard Medical School, Boston, is investigating how neuronal activity can regulate gene expression through a potentially novel mechanism in the developing brain, called RNA turnover. This mechanism may enable gene expression to be rapidly and locally controlled at individual connections between neurons based on neuronal activity. Evidence shows that neuronal activity may contribute to pediatric malignant glioma brain tumors. Dr. Duffy aims to characterize this process and identify new therapeutic targets for pediatric brain cancer.
Erin E. Duffy, PhD, with her sponsor Michael E. Greenberg, PhD, at Harvard Medical School, Boston, is investigating how neuronal activity can regulate gene expression through a potentially novel mechanism in the developing brain, called RNA turnover. This mechanism may enable gene expression to be rapidly and locally controlled at individual connections between neurons based on neuronal activity. Evidence shows that neuronal activity may contribute to pediatric malignant glioma brain tumors. Dr. Duffy aims to characterize this process and identify new therapeutic targets for pediatric brain cancer.
 Courtney Ellison, PhD, with her sponsors Joshua Shaevitz, PhD, and Zemer Gitai, PhD, at Princeton University, Princeton, is investigating how single bacterial cells join together to form complex, multicellular structures called biofilms. Biofilms protect bacterial cells from antibiotics and antimicrobial agents, making them difficult to eliminate. Some biofilm-forming species may cause certain cancers, and biofilms of infectious bacteria threaten immunocompromised patients such as those undergoing chemotherapy. Dr. Ellison focuses on bacterial appendages called type IV pili that play a crucial role in biofilm formation. Understanding the role of pili and their contribution to biofilm progression may lead to novel therapies to eliminate biofilms.
Courtney Ellison, PhD, with her sponsors Joshua Shaevitz, PhD, and Zemer Gitai, PhD, at Princeton University, Princeton, is investigating how single bacterial cells join together to form complex, multicellular structures called biofilms. Biofilms protect bacterial cells from antibiotics and antimicrobial agents, making them difficult to eliminate. Some biofilm-forming species may cause certain cancers, and biofilms of infectious bacteria threaten immunocompromised patients such as those undergoing chemotherapy. Dr. Ellison focuses on bacterial appendages called type IV pili that play a crucial role in biofilm formation. Understanding the role of pili and their contribution to biofilm progression may lead to novel therapies to eliminate biofilms.
 Keelan Z. Guiley, PhD [HHMI Fellow], with his sponsor Kevan M. Shokat, PhD, at the University of California, San Francisco, is focusing on the tumor suppressor protein p53, which is inactivated in half of all cancers, making it the most frequently mutated gene in cancer patients. Normally, when DNA is damaged, p53 stops cell division to allow the cell to repair acquired mutations. Without this safety measure, cells continue to divide and become cancerous. Dr. Guiley aims to develop a novel small molecule drug approach to target a common mutation in p53 and restore its function. These studies have the potential to benefit patients with many different types of cancer.
Keelan Z. Guiley, PhD [HHMI Fellow], with his sponsor Kevan M. Shokat, PhD, at the University of California, San Francisco, is focusing on the tumor suppressor protein p53, which is inactivated in half of all cancers, making it the most frequently mutated gene in cancer patients. Normally, when DNA is damaged, p53 stops cell division to allow the cell to repair acquired mutations. Without this safety measure, cells continue to divide and become cancerous. Dr. Guiley aims to develop a novel small molecule drug approach to target a common mutation in p53 and restore its function. These studies have the potential to benefit patients with many different types of cancer.
 Julia Su Zhou Li, PhD, with her sponsor Don W. Cleveland, PhD, at the University of California, San Diego, focuses on how cells become cancerous when they have an abnormal number of chromosomes or broken parts of a chromosome. The centromere, which joins two arms of a chromosome, is essential for faithful chromosome segregation during cell division and genome stability. When chromosomes fail to be delivered correctly to each new cell, the abnormal chromosomes may form “neocentromeres” which have been discovered in developmental disorders and cancer. Dr. Li is developing tools to examine and manipulate these neocentromeres, which may lead to a better understanding of how cancer cells evolve and potentially novel anti-tumor strategies.
Julia Su Zhou Li, PhD, with her sponsor Don W. Cleveland, PhD, at the University of California, San Diego, focuses on how cells become cancerous when they have an abnormal number of chromosomes or broken parts of a chromosome. The centromere, which joins two arms of a chromosome, is essential for faithful chromosome segregation during cell division and genome stability. When chromosomes fail to be delivered correctly to each new cell, the abnormal chromosomes may form “neocentromeres” which have been discovered in developmental disorders and cancer. Dr. Li is developing tools to examine and manipulate these neocentromeres, which may lead to a better understanding of how cancer cells evolve and potentially novel anti-tumor strategies.
 Kaixian Liu, PhD, with his sponsors Scott N. Keeney, PhD, and Shixin Liu, PhD, at Memorial Sloan Kettering Cancer Center, New York, is combining single-molecule fluorescence and force spectroscopy to study dynamic interactions between meiotic double-strand break (DSB) proteins and DNA. Meiotic recombination, which shuffles genes during the formation of gametes, initiates with DSBs that are generated by the protein Spo11. Spo11 and its partner proteins ensure that DSBs occur at the right chromosome sites and at the right time. Dysregulated DSBs lead to chromosome instability, a hallmark of cancer cells. Dr. Liu’s study will elucidate the dynamics of DSB formation during meiosis, which will shed light on cancer initiation and pave the way for new therapeutic alternatives.
Kaixian Liu, PhD, with his sponsors Scott N. Keeney, PhD, and Shixin Liu, PhD, at Memorial Sloan Kettering Cancer Center, New York, is combining single-molecule fluorescence and force spectroscopy to study dynamic interactions between meiotic double-strand break (DSB) proteins and DNA. Meiotic recombination, which shuffles genes during the formation of gametes, initiates with DSBs that are generated by the protein Spo11. Spo11 and its partner proteins ensure that DSBs occur at the right chromosome sites and at the right time. Dysregulated DSBs lead to chromosome instability, a hallmark of cancer cells. Dr. Liu’s study will elucidate the dynamics of DSB formation during meiosis, which will shed light on cancer initiation and pave the way for new therapeutic alternatives.
 Jon McGinn, PhD, with his sponsors Rebecca Lamason, PhD, and Michael Laub, PhD, at the Massachusetts Institute of Technology, Cambridge, studies how bacterial pathogens sense and manipulate their human hosts. Dr. McGinn is focusing on the tick-borne bacterial pathogen Rickettsia parkeri, which can only survive within eukaryotic host cells. By uncovering novel interactions between host and pathogen, his work may reveal new insights into how human cells work and what goes awry in disease states. He is also developing tools to manipulate key virulence pathways in Rickettsia parkeri that can be used to transform the bacteria into a vehicle for delivering antigens or new drugs directly to cancer cells.
Jon McGinn, PhD, with his sponsors Rebecca Lamason, PhD, and Michael Laub, PhD, at the Massachusetts Institute of Technology, Cambridge, studies how bacterial pathogens sense and manipulate their human hosts. Dr. McGinn is focusing on the tick-borne bacterial pathogen Rickettsia parkeri, which can only survive within eukaryotic host cells. By uncovering novel interactions between host and pathogen, his work may reveal new insights into how human cells work and what goes awry in disease states. He is also developing tools to manipulate key virulence pathways in Rickettsia parkeri that can be used to transform the bacteria into a vehicle for delivering antigens or new drugs directly to cancer cells.
 Colleen N. McLaughlin, PhD [HHMI Fellow], with her sponsor Liqun Luo, PhD, at Stanford University, Stanford, is using the developing nervous system to study metastasis, the primary cause of cancer-related fatalities. In metastasis, cell surface and secreted molecules enable cells to travel through diverse environments and invade distant tissues. Likewise, growing axons in the developing nervous system use similar sets of cell surface proteins to traverse long distances to form precise connections with their synaptic partner cells. Dr. McLaughlin aims to define the mechanisms used by cell surface proteins to promote axon targeting, which will provide critical insight into how these molecules are harnessed by malignant cells during metastasis.
Colleen N. McLaughlin, PhD [HHMI Fellow], with her sponsor Liqun Luo, PhD, at Stanford University, Stanford, is using the developing nervous system to study metastasis, the primary cause of cancer-related fatalities. In metastasis, cell surface and secreted molecules enable cells to travel through diverse environments and invade distant tissues. Likewise, growing axons in the developing nervous system use similar sets of cell surface proteins to traverse long distances to form precise connections with their synaptic partner cells. Dr. McLaughlin aims to define the mechanisms used by cell surface proteins to promote axon targeting, which will provide critical insight into how these molecules are harnessed by malignant cells during metastasis.
 Ryan H. Moy, MD, PhD [Robert Black Fellow], with his sponsor Sohail Tavazoie, MD, PhD, at The Rockefeller University, New York, is focusing on key genes and pathways that control metastasis of colorectal cancer to the liver. Dr. Moy’s research could potentially lead to new treatment strategies that can block or delay metastatic progression of colorectal cancer and other gastrointestinal malignancies. Understanding this process at the molecular level has the potential to impact patient survival.
Ryan H. Moy, MD, PhD [Robert Black Fellow], with his sponsor Sohail Tavazoie, MD, PhD, at The Rockefeller University, New York, is focusing on key genes and pathways that control metastasis of colorectal cancer to the liver. Dr. Moy’s research could potentially lead to new treatment strategies that can block or delay metastatic progression of colorectal cancer and other gastrointestinal malignancies. Understanding this process at the molecular level has the potential to impact patient survival.
 Ysbrand M. Nusse, PhD [Robert Black Fellow], with his sponsor Paul Kubes, PhD, at the University of Calgary, Calgary, studies how immune cells contribute to liver regeneration after injury. Injuries to mammalian tissues are typically repaired through a stepwise process of inflammation and debris clearance, followed by proliferation of progenitor cells and tissue reorganization. During chronic injury, this process can malfunction leading to excessive inflammation, uncontrolled tissue growth and cancer initiation. Dr. Nusse is investigating the role of eosinophils, a type of disease-fighting white blood cell, in liver damage and repair using mouse genetics and imaging within living tissues. This project aims to uncover how liver cancers arise from chronic liver damage and may also reveal insight into other forms of cancer.
Ysbrand M. Nusse, PhD [Robert Black Fellow], with his sponsor Paul Kubes, PhD, at the University of Calgary, Calgary, studies how immune cells contribute to liver regeneration after injury. Injuries to mammalian tissues are typically repaired through a stepwise process of inflammation and debris clearance, followed by proliferation of progenitor cells and tissue reorganization. During chronic injury, this process can malfunction leading to excessive inflammation, uncontrolled tissue growth and cancer initiation. Dr. Nusse is investigating the role of eosinophils, a type of disease-fighting white blood cell, in liver damage and repair using mouse genetics and imaging within living tissues. This project aims to uncover how liver cancers arise from chronic liver damage and may also reveal insight into other forms of cancer.
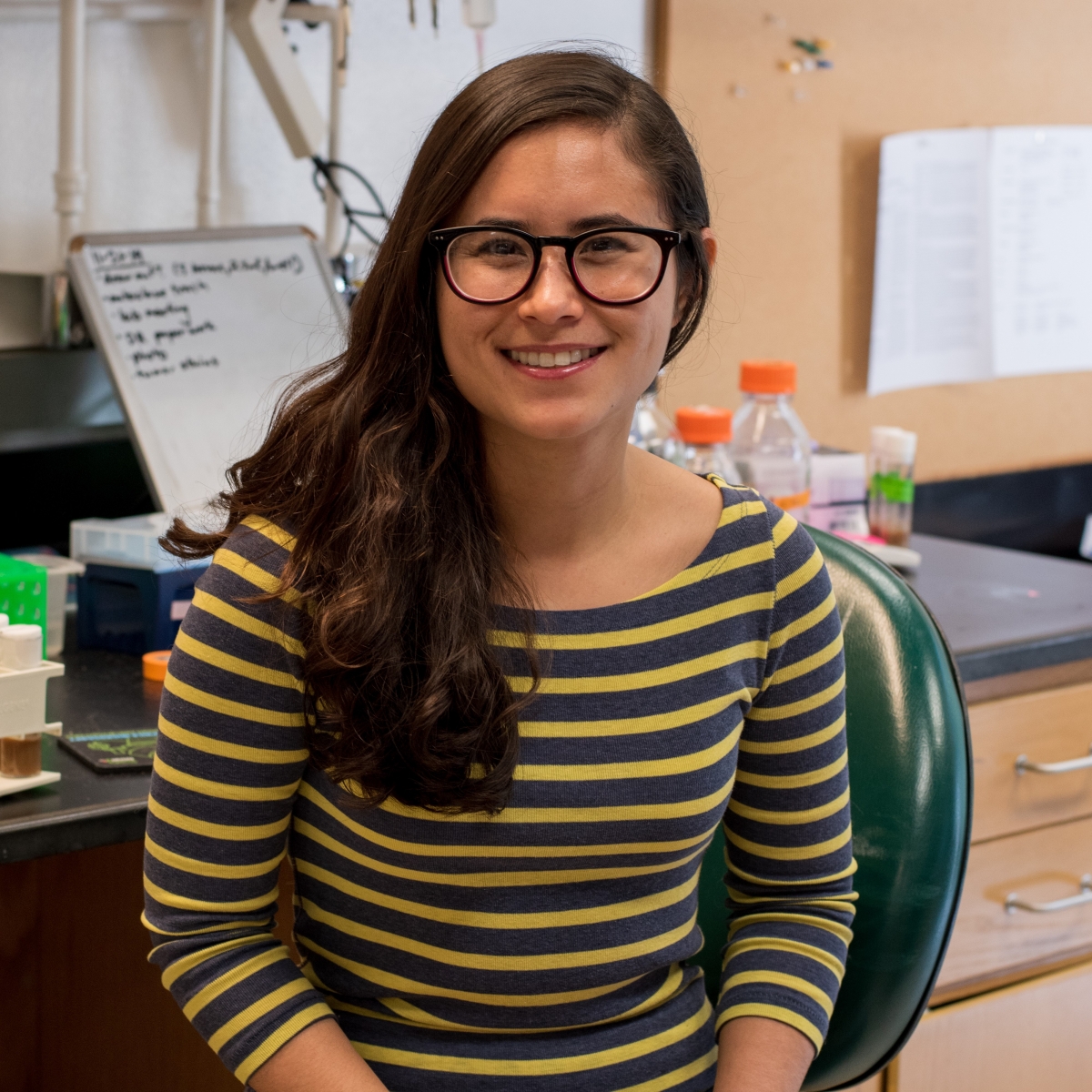 Katy Ong, PhD [The Mark Foundation for Cancer Research Fellow], with her sponsor David Bilder, PhD, at the University of California, Berkeley, is investigating the molecular basis of paraneoplastic syndromes, which occur when a cancer causes unusual symptoms due to hormones produced by the tumor or antibodies produced by the immune system. They can affect the function of various distant tissues and organs in cancer patients, with deadly consequences. Dr. Ong is utilizing a new genetic tumor model in Drosophila that simulates many human paraneoplastic disorders: cachexia, immune dysfunction and early lethality. She aims to uncover how tumor cells impose physiological changes in host tissues at a distance with the hope of uncovering footholds for novel treatments.
Katy Ong, PhD [The Mark Foundation for Cancer Research Fellow], with her sponsor David Bilder, PhD, at the University of California, Berkeley, is investigating the molecular basis of paraneoplastic syndromes, which occur when a cancer causes unusual symptoms due to hormones produced by the tumor or antibodies produced by the immune system. They can affect the function of various distant tissues and organs in cancer patients, with deadly consequences. Dr. Ong is utilizing a new genetic tumor model in Drosophila that simulates many human paraneoplastic disorders: cachexia, immune dysfunction and early lethality. She aims to uncover how tumor cells impose physiological changes in host tissues at a distance with the hope of uncovering footholds for novel treatments.
 Nikit Patel, PhD, with his sponsor Allon Klein, PhD, at Harvard Medical School, Boston, is focusing on hematopoiesis, the process by which stem cells in the bone marrow differentiate into all the blood and immune cells in our bodies. Breakdown of this process is linked to cancers including myelomas and leukemias. Dr. Patel aims to determine critical components driving hematopoiesis by studying this process in different vertebrate animals and comparing evolutionarily conserved regulators. He will then use gene-editing methods to test if these regulators play a role in mammalian hematopoiesis. This work has the potential to identify new therapeutic targets and novel strategies for combatting blood-related cancers.
Nikit Patel, PhD, with his sponsor Allon Klein, PhD, at Harvard Medical School, Boston, is focusing on hematopoiesis, the process by which stem cells in the bone marrow differentiate into all the blood and immune cells in our bodies. Breakdown of this process is linked to cancers including myelomas and leukemias. Dr. Patel aims to determine critical components driving hematopoiesis by studying this process in different vertebrate animals and comparing evolutionarily conserved regulators. He will then use gene-editing methods to test if these regulators play a role in mammalian hematopoiesis. This work has the potential to identify new therapeutic targets and novel strategies for combatting blood-related cancers.
 Veronika Shoba, PhD, with her sponsors Amit Choudhary, PhD, and Stuart L. Schreiber, PhD, at the Broad Institute, Cambridge, aims to develop a new class of small molecules that will target transcription factors, which regulate gene expression. These proteins are mutated in many cancers but are considered undruggable because current compounds fail to block the interaction between transcription factors and their binding partners. Dr. Shoba aims to disrupt these functional interactions by recruiting enzymes called kinases found in the human body to chemically modify the transcription factors and thus inhibit oncogenic activity. This strategy has the potential to diversify cancer-targeting approaches and become an essential tool in drug development.
Veronika Shoba, PhD, with her sponsors Amit Choudhary, PhD, and Stuart L. Schreiber, PhD, at the Broad Institute, Cambridge, aims to develop a new class of small molecules that will target transcription factors, which regulate gene expression. These proteins are mutated in many cancers but are considered undruggable because current compounds fail to block the interaction between transcription factors and their binding partners. Dr. Shoba aims to disrupt these functional interactions by recruiting enzymes called kinases found in the human body to chemically modify the transcription factors and thus inhibit oncogenic activity. This strategy has the potential to diversify cancer-targeting approaches and become an essential tool in drug development.
 Kouki Touhara, PhD [Robert A. Swanson Family Fellow], with his sponsor David Julius, PhD, at the University of California, San Francisco, is focusing on enteroendocrine cells which are found in the wall of the gut and secrete hormones that regulate glucose levels, food intake and stomach emptying. Abnormal activity of these cells often causes common gastrointestinal disorders such as irritable bowel syndrome and carcinoid tumors. Dr. Touhara aims to elucidate interactions of the enteroendocrine cells with the enteric nervous system which controls gut motility and communicates with the brain. This research may lead to new drug targets and treatments for disorders of the gut.
Kouki Touhara, PhD [Robert A. Swanson Family Fellow], with his sponsor David Julius, PhD, at the University of California, San Francisco, is focusing on enteroendocrine cells which are found in the wall of the gut and secrete hormones that regulate glucose levels, food intake and stomach emptying. Abnormal activity of these cells often causes common gastrointestinal disorders such as irritable bowel syndrome and carcinoid tumors. Dr. Touhara aims to elucidate interactions of the enteroendocrine cells with the enteric nervous system which controls gut motility and communicates with the brain. This research may lead to new drug targets and treatments for disorders of the gut.







