Demystifying the process of DNA repair
Like a leaky gas pipe in an apartment building, failure to repair DNA damage can have disastrous consequences, including the introduction of cancer-causing mutations. This is why our cells have complex mechanisms for recognizing and repairing broken DNA strands before too much damage has been done. In a big-picture sense, we know how DNA repair works: proteins responsible for sensing damage activate a cascade of other proteins, depending on the nature and location of the problem. But a granular understanding of this process, including which genes are involved, continues to elude scientists.
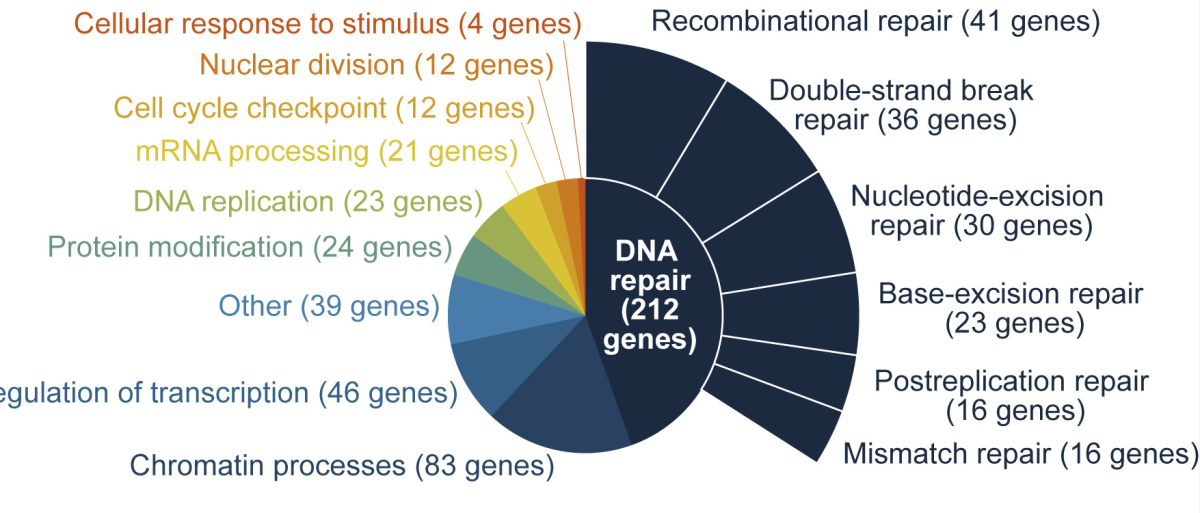
Seeking to “map the genetic landscape” of DNA repair, former Damon Runyon Fellows Britt Adamson, PhD, and Jeffrey A. Hussmann, PhD, (at Princeton University and University of California, San Francisco, respectively) have developed a new computational tool, known as Repair-seq, to sequence thousands of DNA repair attempts. In a recent study, the team used the gene editing system CRISPR/Cas9 to knock out 476 repair-associated genes and snip DNA strands at targeted sites. They then employed Repair-seq to assess how eliminating the function of these 476 genes impacted the process of DNA repair.
With the data generated by Repair-seq, the team was able to parse the roles of specific genes in the cell’s complicated repair machinery. They identified, for example, 23 genes responsible for excising erroneous bases, 36 genes needed for mending double-strand breaks, and 16 genes involved in mismatch repair, or the re-aligning of complementary base pairs. Interestingly, the team found that mutations with similar sequences can arise from distinct (faulty) repair mechanisms, revealing relationships between genes not previously connected.
Beyond elucidating the genetic underpinnings of mutations that arise from DNA damage, Repair-seq also makes it possible to study how cells respond, in both intended and unintended ways, to DNA damage induced by genome editing tools. "We've known for a long time that the mechanisms involved in fixing broken DNA are essential for genome editing because to change the sequence of DNA you first have to break it," Dr. Adamson told Science Daily. She envisions scientists using Repair-seq to “take a detailed picture of what genome editors are doing inside cells” and identify “design principles” to improve genome-editing techniques. Given how many targeted therapies rely on the power of genome editing, Repair-seq may soon deserve a place next to CRISPR in the annals of precision medicine.
This research was published in Cell.
